Inverse CeOx/Co Catalysts for CO₂ Methanation
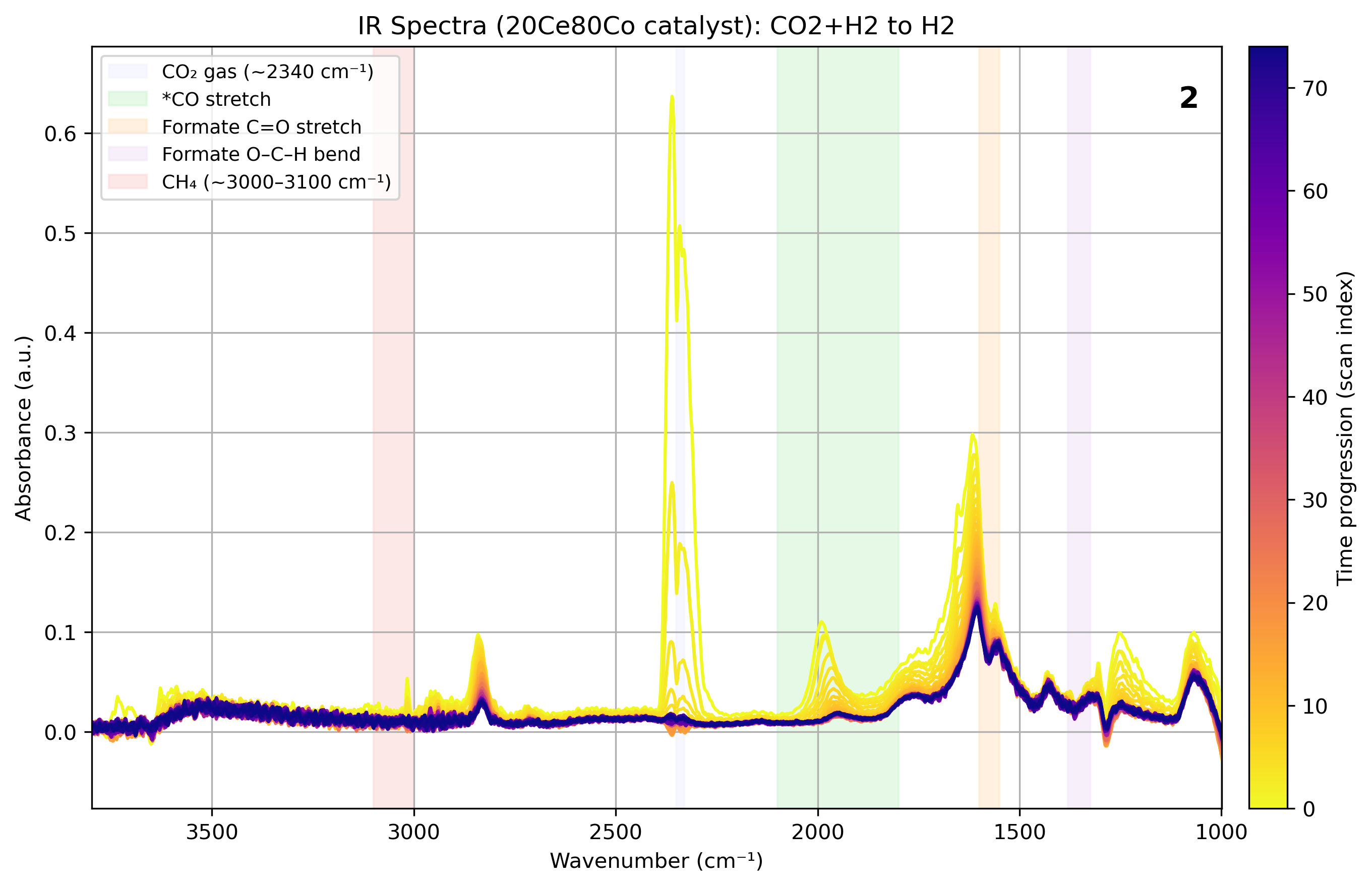
This project investigates flame-spray synthesized inverse CeOx/Co catalysts for low-temperature CO₂ methanation. Characterization techniques including H₂-TPR, CO chemisorption, XRD, and operando IR spectroscopy were used to analyze structure–activity relationships and determine reaction mechanisms.
Results highlight ceria’s promotional role in cobalt dispersion, oxygen vacancy formation, and formate-mediated methanation. The 20Ce80Co catalyst exhibited the highest activity and CH₄ selectivity, with operando IR revealing a sequential CO₂ → CO → CH₄ pathway.